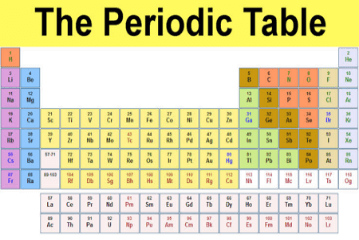
The periodic table of elements has come fine as one of the most famous concepts of science over the years. This table was developed over the years by a man known as Dmitri Mendeleev and Lother Meyer around the year 1869. This table contains a number of elements present in the world, even the less natural occurring ones. It goes to prove that the universe also posses the same compounds and components as other planets and space itself. This table has an order from groups that go from top to bottom or orders from elements that go from top to bottom.

In the universe, earth and outer space, all energy and matter we observe are made and caused by these elements. These elements are substances that are made up of atoms and have a similar amount of protons present.
Atoms
Atom is a word that was derived from a Greek word. It means invisible and it is the smallest and most indivisible part of any element. If you take a part of an element such as silver and have it divided into two parts, they are still considered as silver. No matter how many times and how many parts you have them divided into, they can and will always remain that particular element.
Let’s say you have a single silver element. This cab ever very difficult to divide but let’s say you do. Once you divide it, it isn’t referred to as single after that. That goes to show that atoms aren’t indivisible by principle but instead become other atoms when they are divided.
Atoms might be small but they play a large part and consist of nothing in a spatial sense. What’s left is just the nucleus and shells. However, this nucleus consists of neutrons and protons which are shells of electrons. They are also more neutral when it comes to electricity and protons are positively charged. Neurons are responsible for the negatively charged electrons which circle around the nucleus as opposite sides attack each other hence the plus and minus attraction.

Properties Of A Periodic Table
Properties of an element on the periodic table are due to the number and arrangement of electrons. It is also important to know that electrons in the outermost shell are very important and they are known as valence electrons. This means that different elements possess different chemical properties but elements present in the same group possess similar properties due to their valence electrons. The number of shells present in an atom depends on the number of electrons although notion shells aren’t not to be taken literally.
The very first element that was thought to occur after the Big Bang was hydros which consist of single protons. The universe became so hot and dense and this made it fuse to form helium and nothing relating to elements happened in a long time. The periodic table shows us all these elements and what they consist of both on earth and outside of it. It is also called the periodic table of elements.